Transgenic Animals Ppt Presentation
Specially for class 11 and neet aspirants.
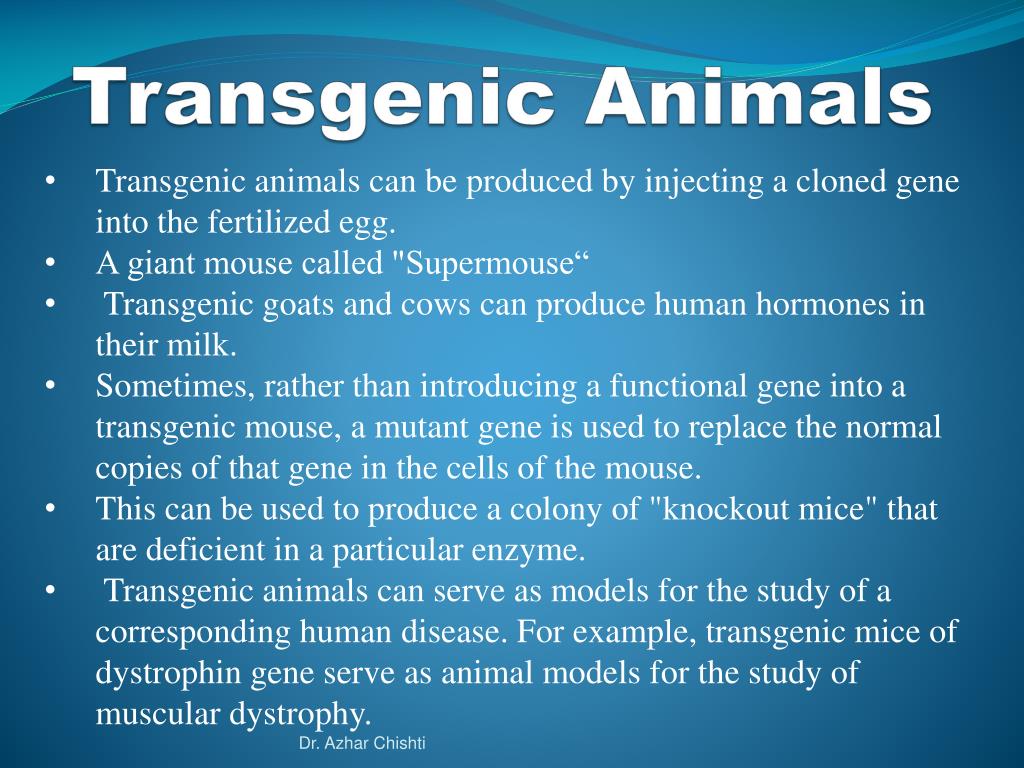
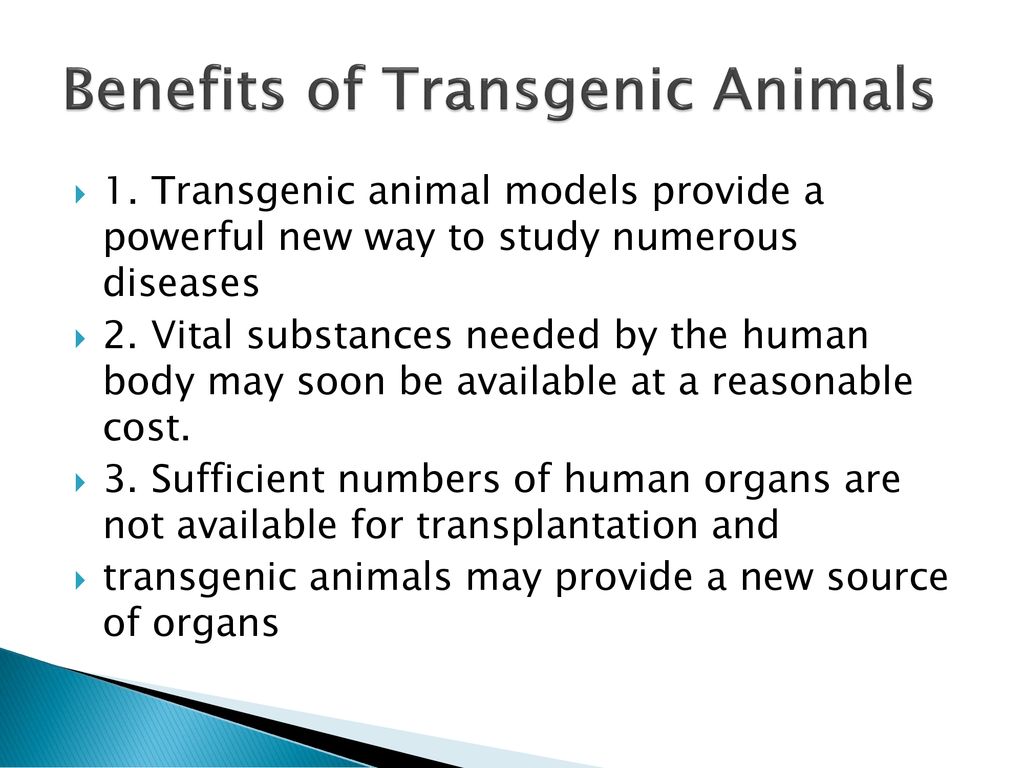
Transgenic animals ppt presentation. Ppt microencapsulation SANA TABASSUM. Other transgenic animals are produced as disease models animals genetically manipulated to exhibit disease symptoms so. Transgenic mouse models have been developed for human genetic diseases such as Alzheimer disease Huntington disease Tumorigenesis Endocrine dysfunction Coronary disease and many others.
This lecture states some mechanisms to develop healthy transgenic animals. These are filariasis chikungunya dengue kala azar and japanese encephalitis. Unfortunately drug regulations forbid public review of all safety evidence until after FDA approvaland the FDA has interpreted that this secrecy also applies to ecological safety.
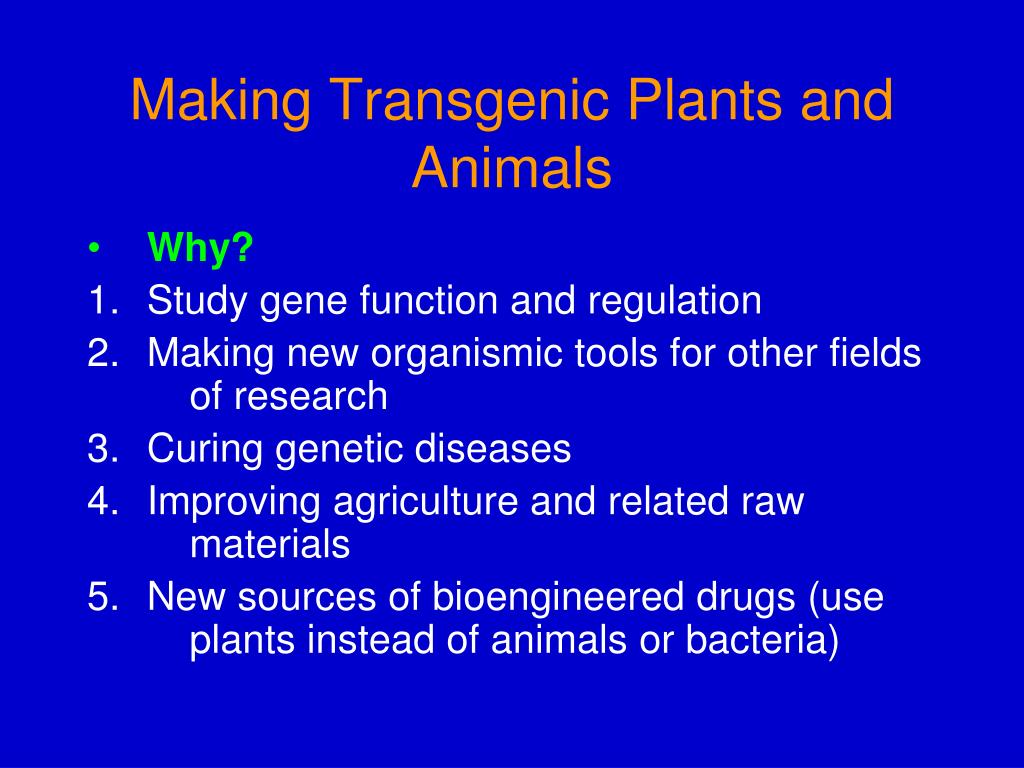
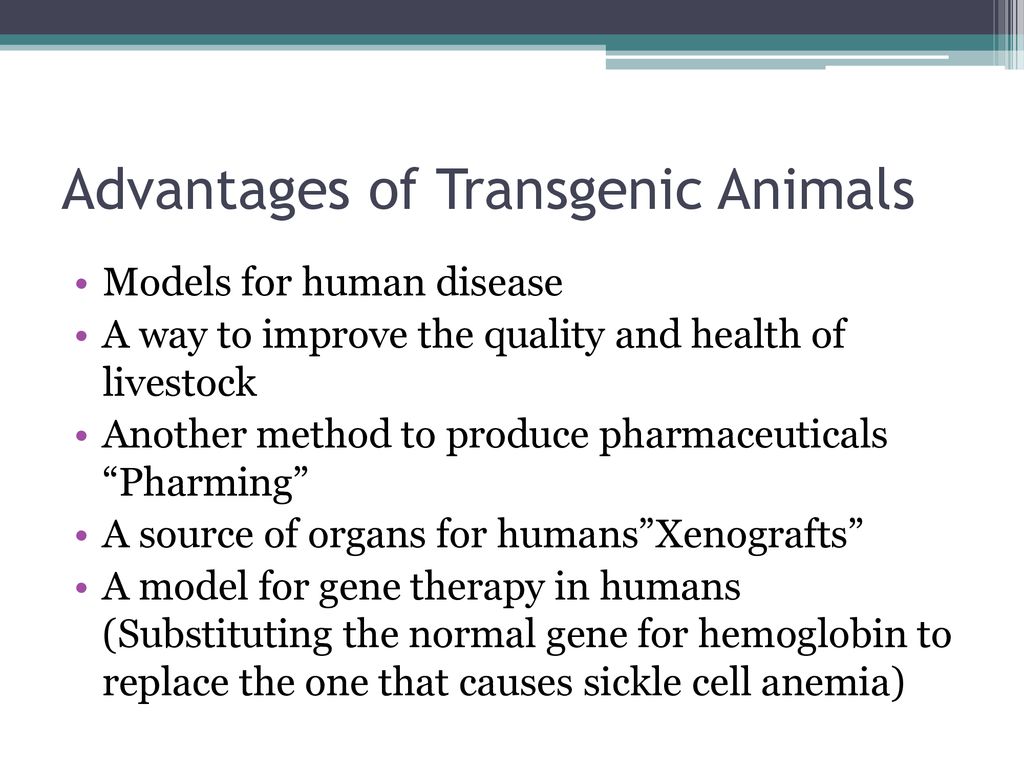
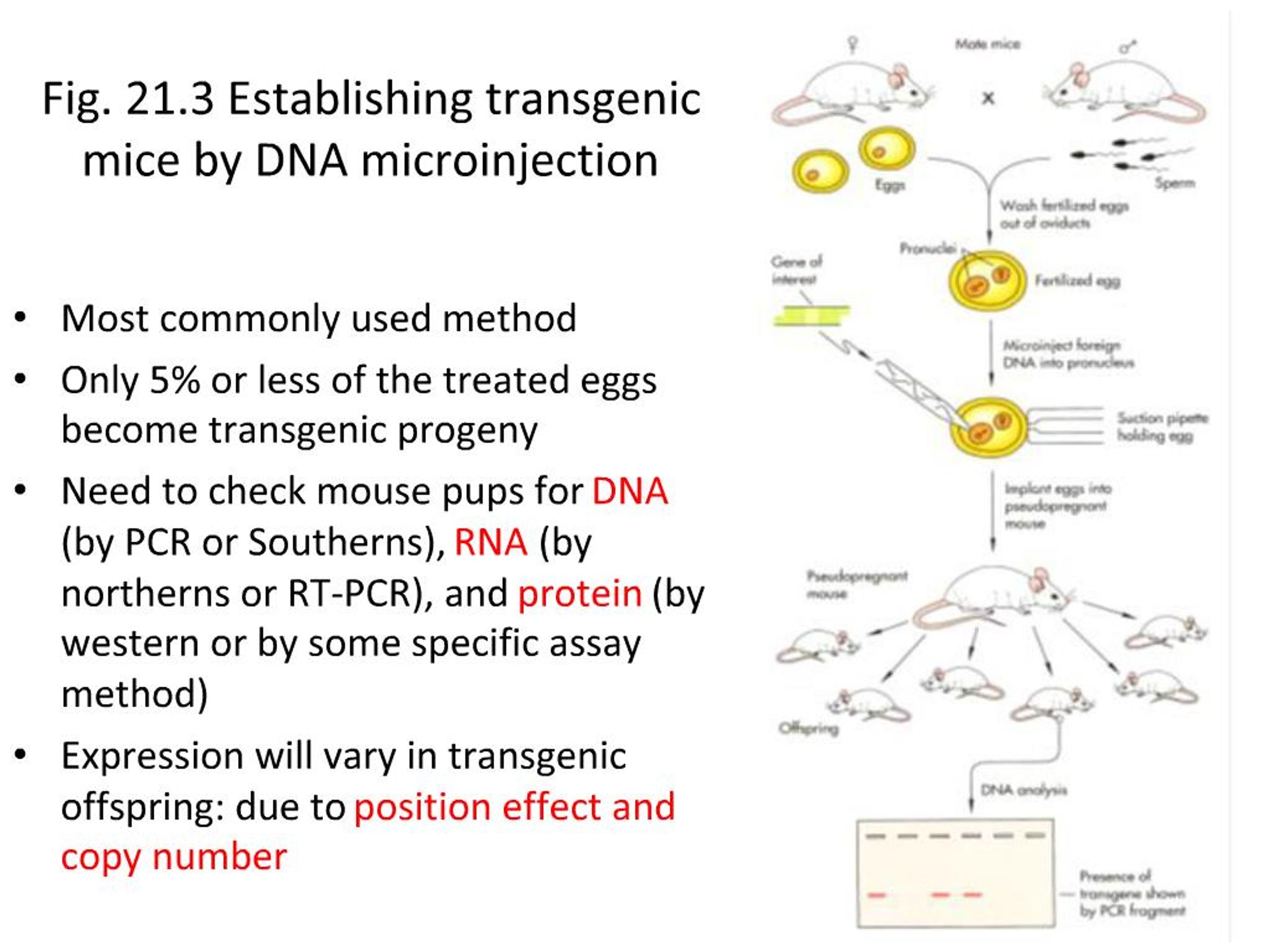
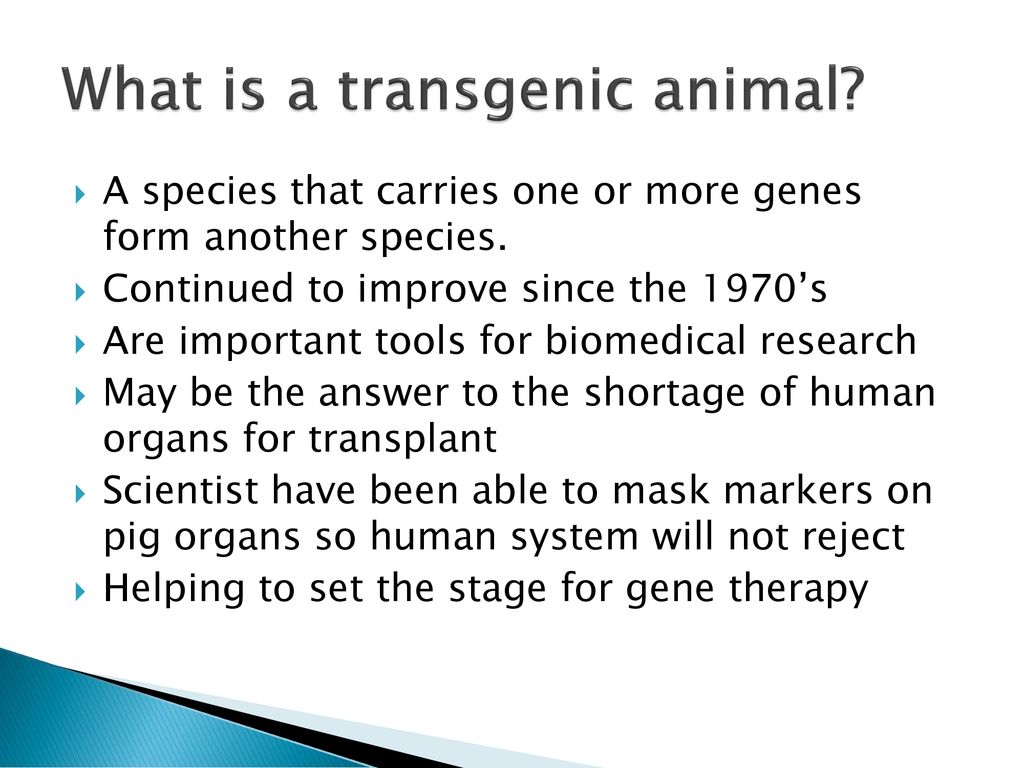
Animal biotechnology is the field to engineer transgenic animals ie animals that carry genes from other species. A transgenic animal is one that carries a foreign gene that has been deliberately inserted into its genome. This lecture explains about the principle behind the transgenic animal production.
The Food and Drug Administration FDA is taking regulatory lead over transgenic fish and other aquatic animalsbased on arguing that GM animals fit the legal definition of a drug. Times New Roman MS Pゴシック Arial Default Design Microsoft Photo Editor 30 Photo Package Recombinant DNA Technology Cloning PowerPoint Presentation Recombinant DNA How to get rDNA PowerPoint Presentation Transfer of rDNA into Cells PowerPoint Presentation Transgenic Animals PowerPoint Presentation Transgenic Plants Gene Therapy DNA Fingerprinting PowerPoint Presentation. Do research directly with animals.
Thus the abilities or the phenotype of the organism or the. Thi ppt is about national vector borne disease control programme. Pros Cons Chetana Tamadaddi.
Transgenic animals and knockout animals 3 main ways to do biological research. Genetically Modified Organisms Genetically modified organisms are organisms with artificially altered DNA. Polymerase chain reaction PCR.
_1602913203_391831-16.jpg)
_1602913203_391831-15.jpg)
_1602913203_391831-4.jpg)
_1602913203_391831-1.jpg)



_1602913203_391831-5.jpg)
_1602913203_391831-2.jpg)

_1602913203_391831-12.jpg)